Introduction:
Stem cell transplantation (SCT) is used to treat multiple malignancies, but a major complication of the procedure is graft versus host disease (GvHD), which is a significant cause of morbidity and death in SCT patients. Methylprednisolone is the first-line therapy of GvHD. Ruxolitinib is a Janus kinase (JAK) inhibitor that can dampen the effect of inflammatory cytokines involved in GvHD and may be used in patients refractory to steroid treatment. In this systematic review and meta-analysis, we assessed the safety and efficacy of Ruxolitinib in steroid-resistant (SR) acute (a) and chronic (c) GvHD.
Methods:
We performed a search on PubMed, Cochrane, Embase, and Web of Science. We used the keywords, "Ruxolitinib" AND "Graft vs Host Disease" from the inception of literature till 7/10/2020. We screened 694 articles and included 1 randomized clinical trial (RCT) (N=309), 4 non-randomized trials (NRCT) (N=232), and 13 observational studies (N=481) in this meta-analysis. We extracted data for efficacy (i-e, OS, CR, ORR) and safety (≥grade 3 treatment related adverse events (TRAE). We excluded case reports, case series, review articles, meta-analysis, and preclinical trials. We used the R programming language (version 4.0.2) to conduct a meta-analysis.
Results:
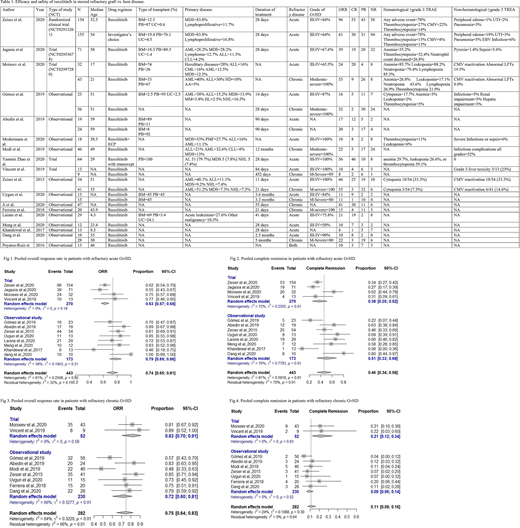
In the 18 included studies (N=1022), Ruxolitinib was used in patients with the age range of 6 months to 70 years. 417 participants had grade III-IV acute GvHD, and 272 participants had moderate to severe chronic GvHD (Table 1).
In an RCT (N=309), patients aged between 12-73 years with SR aGVHD were randomized 1:1 to receive either Ruxolitinib or physician's choice drug. Overall response rate (ORR) and complete response (CR) were significantly higher in the Ruxolitinib group as compared to the physician's choice drug. The results were consistent for all grades of GvHD. Grade 3 or higher treatment-related adverse events (TRAEs) were 78% in the two groups. 22% of the patients died of GvHD related adverse events in the Ruxolitinib group vs. 25% of the patients in the control group.
In 12 clinical trials and observational studies, among SR GvHD patients (N=443), pooled ORR was 0.74 (CI=0.65-0.81, I2=61%) with Ruxolitinib treatment. Similarly, pooled CR was 0.45 (CI=0.34-0.68, I2=81%). The most common adverse events were cytopenias, viral reactivation, and infections. (Fig 1, 2) In 9 early phase trials and observational studies (N=282) in SR- chronic GvHD patients, pooled ORR and pooled CR were 0.75 (CI=0.64-0.83, I2=64%) and 0.11 (CI=0.08-0.16, I2=0), respectively. The most common adverse events were cytopenias and viral reactivation (Fig 3, 4).
In a non-randomized trial (N=64), Ruxolitinib was used in combination with Etanercept for SR- acute GvHD patients. The ORR and CR were 87.5% and 72%, respectively. High rates of hematological adverse events and infections were reported in these patients. In an observational study (N=18), Ruxolitinib was used in combination with Extracorporeal Photopheresis (ECP). The combination was well-tolerated, and ORR and CR were 55% and 44%, respectively (Table 1).
Conclusion:
Ruxolitinib was well tolerated by patients with acute or chronic SR-GvHD. Ruxolitinib showed a higher efficacy compared to the physician's choice drug in SR acute GvHD. Ruxolitinib was also effective in SR cGvHD patients. The addition of etanercept to Ruxolitinib increased the efficacy in SR-acute GvHD patients but resulted in an increased incidence of infections. Ruxolitinib with ECP was effective and well tolerated in SR-acute GvHD. Additional multicenter, randomized, double-blind clinical trials are needed to confirm these results.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract